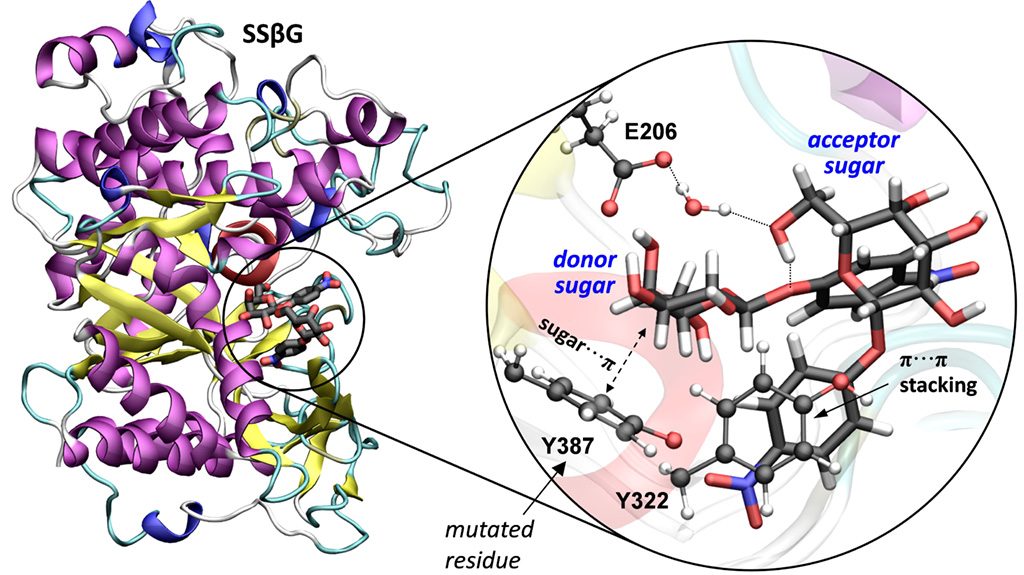
Sugar or carbohydrate synthesis is important for the development of diagnostic tests, vaccines and new drugs. Conventional chemical synthesis in the laboratory is usually expensive and cumbersome; therefore lots of efforts have been made over the last years to do so with enzymes, which are natural catalysts. However, the enzymes that synthetize sugars (called glycosyltransferases) are hard to express and usually use expensive substrates (nucleotide sugars). Now the teams of the University of Barcelona (C. Rovira) and Oxford (Benjamin G. Davis) have found the way to modify a robust glycosidase enzyme, which normally degrades carbohydrates (by catalyzing the hydrolysis of glycosidic bonds) so that it acts reversely and synthetizes carbohydrates. The modification consisted on a single mutation of one of the catalytic residues. Using properly activated carbohydrate substrates, synthesis instead of hydrolysis was exclusively observed. Moreover, such synthesis takes place with a chemical reaction that had not been observed in glycosidases yet, the so called front-face or SN i-like reaction, which the team of Rovira uncovered in 2011 (see previous Highlight) for glycosyltransferases.
To prove that the reaction follows a front-face mechanism, Rovira’s team modeled the binding of two carbohydrate molecules in the active site of the engineered enzyme using free energy enhanced sampling methods and modern supercomputers (MareNostrum at BSC-CNS). Afterwards, they obtained the chemical reaction mechanism using quantum mechanics/molecular mechanics (QM/MM) approaches. The computed free energy landscape of the reaction confirmed that the engineered enzyme had changed its catalytic mechanism from SN2 to a SNi type of reaction.
The advantage of this reaction in glycosidases is that it makes the synthesis in a clean way, without hydrolysis residues and using economically viable substrates (activated sugars). In the study, the synthesis was carried out with a specific enzyme, the Sulfolobus solfataricus β-glycosidase (SSβG), but it can be applied to other enzymes using a similar engineering method, opening new possibilities for carbohydrate biosynthesis.
Reference