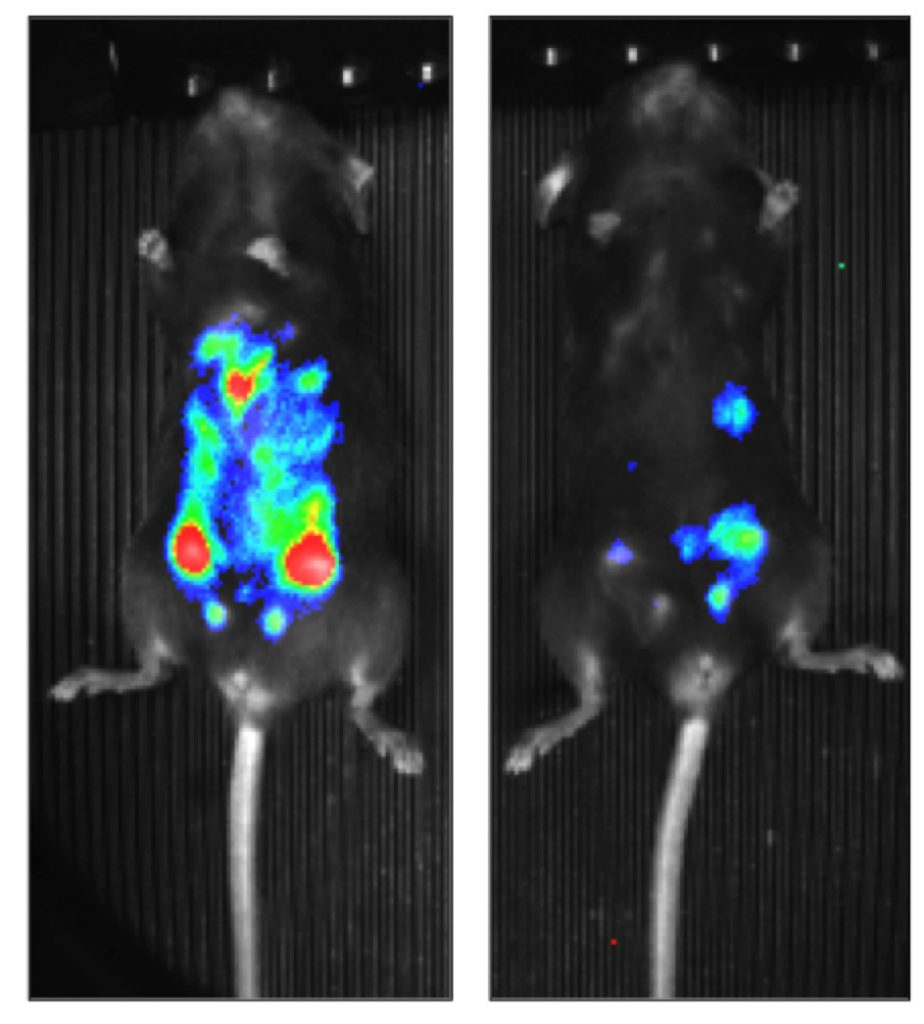
The growth of a tumor depends not only on its cancer cells but also on their interaction with normal cells that form the “tumor microenvironment.” Researchers in the Regulation of Gene Expression Group at IDIBAPS, have identified a protein, ZEB1, that causes the body’s own immune cells to collaborate with cancer cells. The study used a mouse model of ovarian cancer, a Zeb1-deficient mouse, and databases of 400 patients with ovarian cancer. ZEB1 expression in both tumor cells and macrophages at the tumor microenvironment promoted tumor growth. ZEB1 activated cancer cells to produce CCL2—a chemokine that attracts macrophages to the tumor environment—and induced macrophages to release MMP9 that promotes cancer cells to acquire a more aggressive phenotype. A reduction of ZEB1 in macrophages not only inhibited their pro-tumor effect but also improved tumor cell response to chemotherapy.
In a parallel study, the same group has found that ZEB1 inhibits their own senescence, a type of cell ageing. For cancer cells to proliferate, they need to first overcome and inhibit their own mechanisms of tumor suppression being senescence one of them. Analysis of more than 1,000 colorectal carcinomas (CRC) revealed that ZEB1 expression in cancer cells is inversely correlated with a senescence signature and associates with poorer prognosis. Using a Zeb1-deficient mouse and a transgenic mouse model of CRC, the study found that ZEB1 triggers a newly identified pathway (DKK1-mut p53-Mdm2-CtBPmacroH2AY) to inhibit senescence in cancer cells and promote tumor growth. A reduction of ZEB1 in CRC cancer cells to just half of their basal levels was enough to trigger senescence and block the progression of premalignant adenomas into invasive carcinomas.
New cancer therapies seek not only to eliminate tumor cells but also to regulate the immune response against cancer. Likewise, the development of drugs that induce tumor cell senescence is one of the most active areas in anti-cancer therapy and these two studies open the door to the design of new therapeutic strategies towards those goals.
References