Animal cells communicate through chemical signals that are essential for normal development and survival. When one such signal reaches a cell, it must be properly decoded to produce the correct response. Consequently, alterations in these signaling events can lead to multiple diseases, including cancer.
We are studying a protein that functions as a nuclear sensor for the interpretation of the Ras-MAPK pathway, the most frequently mutated signaling pathway in human cancer. We identified this protein in the fruit fly Drosophila and dubbed it Capicua –the Catalan for ‘head and tail’– because embryos that lack Capicua function develop only a head and a tail without the normal middle body regions. The role of Capicua in Ras-MAPK signaling has been conserved throughout evolution and, accordingly, Capicua acts as a tumor suppressor in humans.
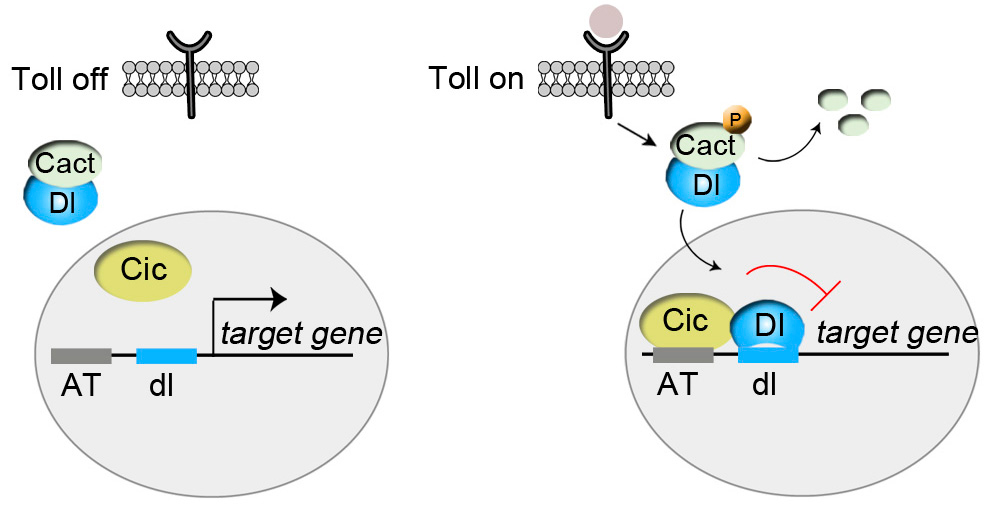
In this work, we have discovered a new role of Capicua in connection with another signaling pathway, the evolutionarily conserved Toll/Interleukin 1 cascade. In this context, Capicua regulates the distinction between dorsal and ventral regions of the Drosophila embryo. It does so by binding to and repressing specific target genes only when Toll/Interleukin 1 signaling becomes active in ventral regions (see the accompanying figure). These results reveal a new paradigm of cell signaling and gene regulation, while at the same time raising the possibility that Ras-MAPK-independent mechanisms may also underlie Capicua function in humans.
Reference