Each chemical reaction that happens in our body would not take place in a timescale compatible with life without the presence of substances called enzymes. Enzymes therefore make life possible. These molecules able to accelerate the chemical reactions are called catalysts. Among all existing catalysts, enzymes are the best ones, as they are capable of accelerating the chemical reactions that take place in our body by many orders of magnitude.
The use of enzymes in industry is, however, still quite limited as they were not optimized for the industrial conditions. Thus, modifying natural enzymes for broadening their scope and applicability in industry is highly appealing given their high potential. Much fundamental and applied research is being carried out to understand the mechanism of action of catalysts and propose modifications to the enzyme structure to improve their efficiency.
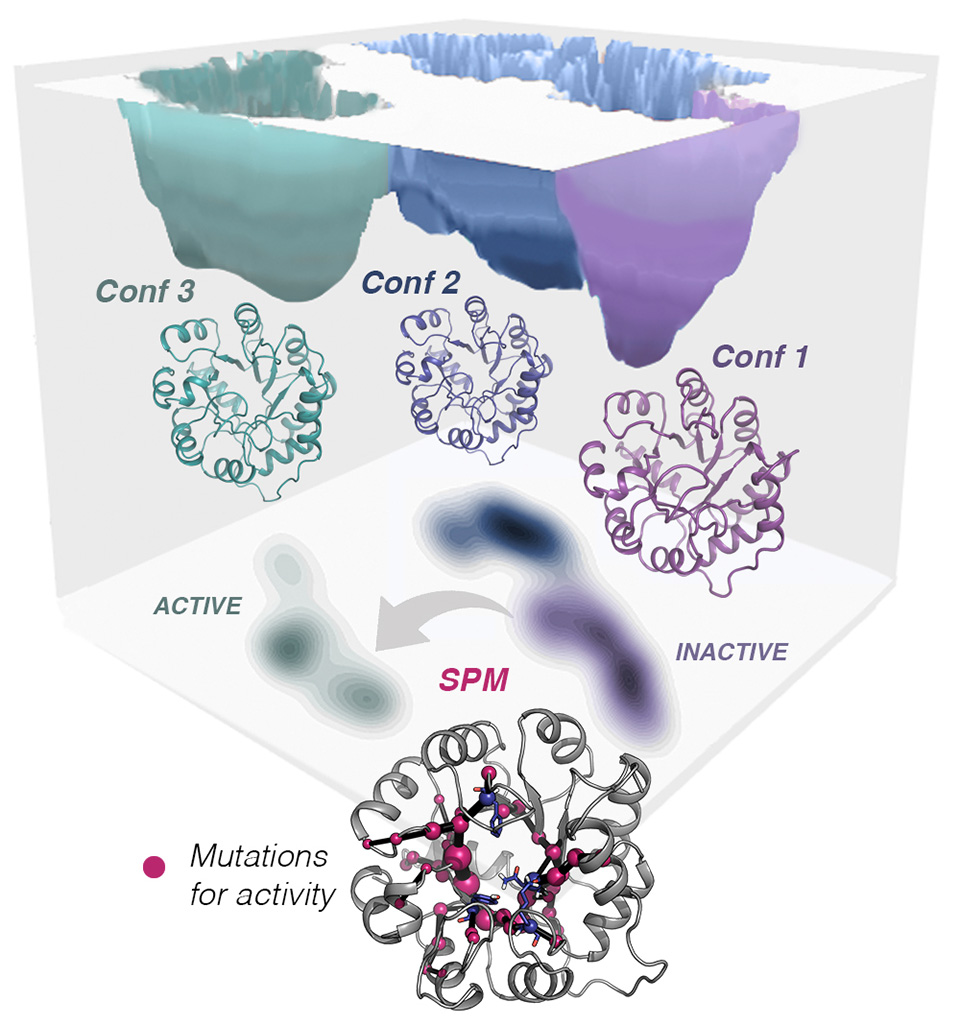
Although it was originally thought that enzymes present a single conformation of their structure, experimental and computational evidences have demonstrated that they can adopt multiple conformations in solution. In fact, enzymes are highly dynamic and can be described as ensembles of differently populated conformations. In the group, we have demonstrated that by introducing mutations to the enzyme, the populations of the accessible conformational states can be tuned, thus enabling emergence of novel function.
We found that careful analysis of the conformational ensemble of enzymes is key for modifying the enzyme ability to accommodate alternative industrially-relevant substrates, and even increase the activity towards some residual promiscuous reactions of interest. We have also developed a new computational tool, that we call Shortest Path Map (SPM), which analyzes the different conformations that the enzyme can adopt and identifies which positions of the enzyme are more important for favoring a desired conformation and enable novel function. Our new developed approach provides the enzyme (re)design field with a rational strategy to determine promising sites for enhancing novel activity through mutation.
Reference