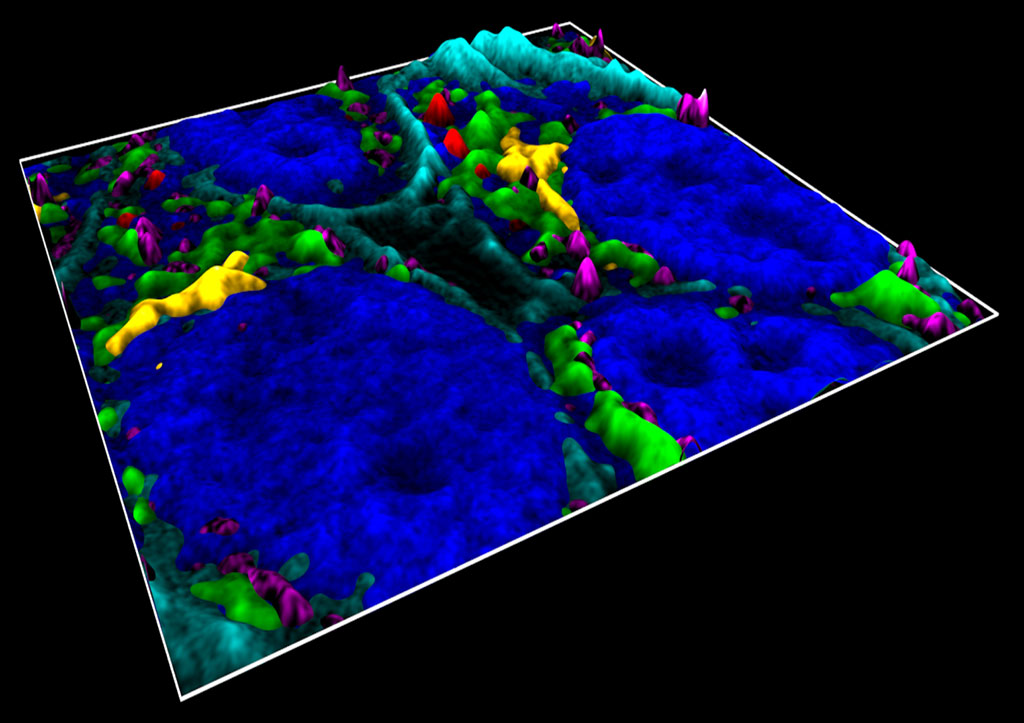
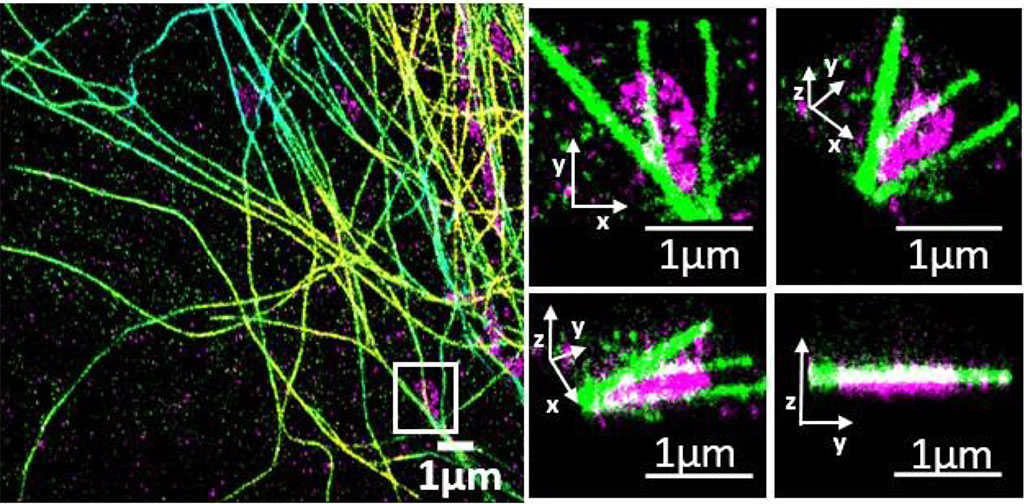
Fluorescence microscopy is one of the most powerful tools employed in life sciences as it allows imaging of different molecular components in cells with high specificity and sensitivity. With the advent of super-resolution microscopy, the possibility of visualizing cellular structures at the nanoscale is now within reach. Therefore, the next grand challenge in biology aims at deciphering spatiotemporal molecular interactions within supramolecular complexes and/or sub-cellular organelles. Although molecular interactions play a vital role in diverse cellular functions, so far they have been poorly addressed as fluorescence microscopy is limited in the number of different labels that can be distinguished simultaneously.
In 2018 we developed a new approach for simultaneous multicolour confocal or multicolour superresolution microscopy that overcomes current challenges in fluorescence imaging and dramatically increases image acquisition time. The method relies on the absorption spectra of fluorescence probes instead of their fluorescence emissions. The method is based on multiplexing optical excitation signals in the frequency domain using single colour-blind detection. Since the spectral information is fully encoded during excitation, the method enables the simultaneous identification of multiple colour channels in a single measurement. We first implemented the method in a confocal configuration and demonstrated simultaneous imaging of six spectrally- and spatially-overlapping fluorophores on fixed cells using four excitation wavelengths. In the case of super-resolution microscopy, we implemented the method in two single molecule localization modalities: DNA-PAINT and STORM. We showed that frequency-multiplexed DNA-PAINT acquires multiple colours at the same time as single-color DNA-PAINT, dramatically improving image acquisition time without compromising the field-of-view or signal throughput.
Our new methodology is fully compatible with live cell imaging and as such it will open up wideranging opportunities for exploring the molecular mechanisms that underpin supramolecular and/or organelle interactions in living cells with unprecedented levels of detail.
References