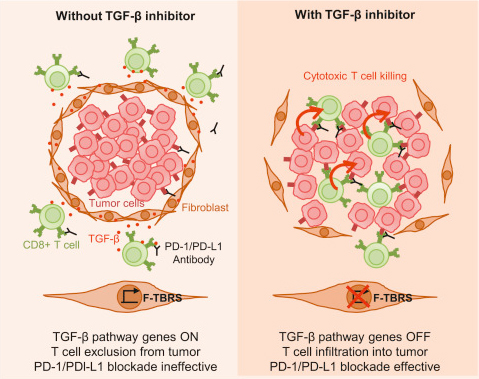
Colorectal cancer (CRC) kills around 700,000 people worldwide every year. The majority of these deaths are the result of the dissemination of the disease to foreign organs. The emergence of new treatment options for patients with late-stage CRC has extended survival periods, yet metastases remain incurable in the majority of patients. Our lab generated for the first time genetically engineered mouse models that develop aggressive human-like CRCs. Analysis of the process of metastasis in these mouse models revealed that tumor cells build an immunoprivileged microenvironment during organ colonization, that promotes T-cell exclusion and blocks the acquisition of an anti-tumor immune effector phenotype. We showed that this mechanism depends on elevated levels of the hormone TGFbeta present in the microenvironment of these metastases. Inhibition of TGF-beta signaling reverts this process and unleashes a potent cytotoxic T cell response against tumor cells, which is long lasting and protects mice from metastatic disease. In mice with progressed liver metastatic disease, blockade of TGF-beta signaling rendered tumors susceptible to anti-PD-1/PD-L1checkpoint immunotherapy. We show that dual TGF-beta/checkpoint therapy cures mice suffering from overt metastatic disease. Our findings suggest that TGF-beta signaling inhibitors may have broad applications to treat patients with advanced CRC. Their efficacy will soon be tested in clinical trials.
References