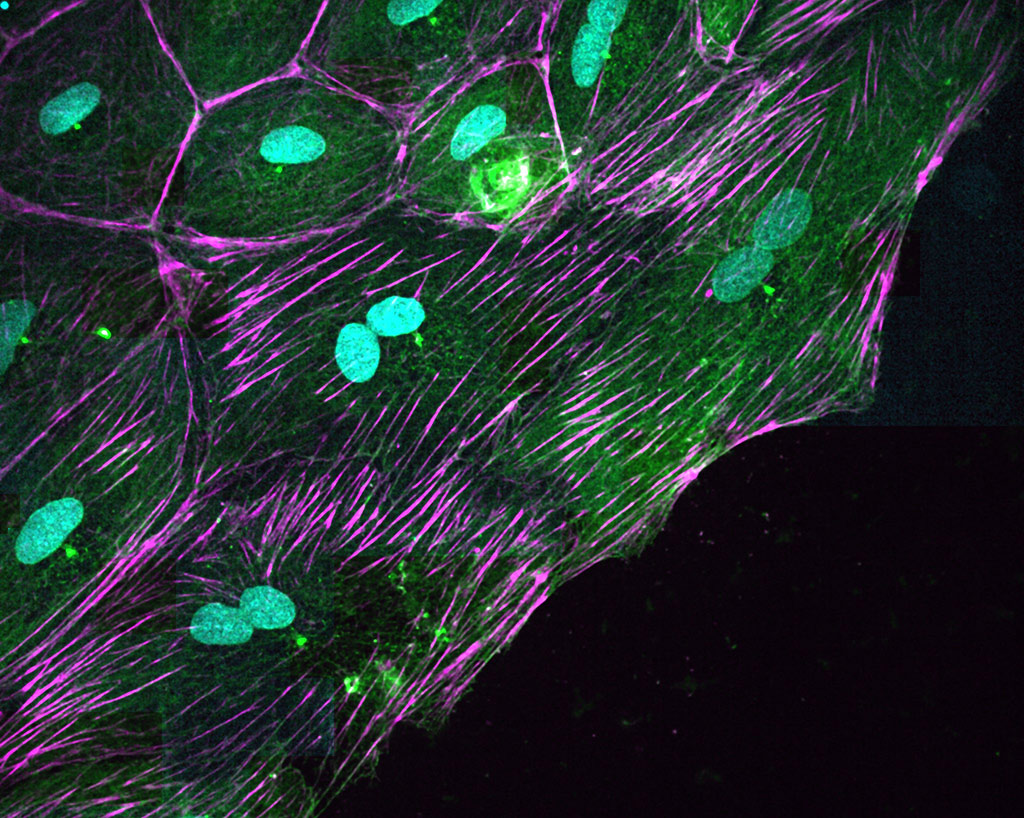
After an acute heart lesion, such as a myocardial infarction, the human heart is unable to regenerate. The adult cardiac cells cannot grow and divide to replace the damaged ones, and the lesion becomes irreversible. But this does not happen in all animals. For example, zebrafish can completely regenerate its heart even after 20% ventricular amputation. This extraordinary regenerative capacity has attracted the attention of researchers from all over the world, who see the range of possibilities that would be opened up if this mechanism of cell regeneration could be applied in human therapies. In collaboration with the team led by Angel Raya, we discovered a surprising mechanism by which zebrafish heart cells move and divide during regeneration. We focused on the epicardium, which is the layer of cells on the outer surface of the heart. Although the epicardium cells represent only a small fraction of the heart’s mass, they play a fundamental role in its regeneration. This tissue is the origin of several of the heart’s cell types, and secretes biochemical signals that tell the cells what they have to do at all times. It’s a kind of regeneration ‘hub’. After a heart lesion, the epicardium cells begin to divide and move en masse to cover the wound. We observed that, during this process, the cells become binucleated: they duplicate the genetic material and separate it into two nuclei, but they are not divided into two independent cells. We discovered that the mechanism by which cells become binucleated has a biomechanical origin. Once DNA has already separated into two nuclei, most animal cells form a contractile ring at its centre. As it contracts, this ring divides the mother cell into two daughter cells. In the case of the heart cells of the zebrafish, our study shows that the ring adheres to the fibres of its environment so that it cannot contract. The result is that the two daughter cells cannot separate despite having duplicated their DNA. Multinucleation is a well-known phenomenon in cancer, because it is a cause of genetic instability. In the zebrafish heart, multinucleation is physiological and may be advantageous.
Reference