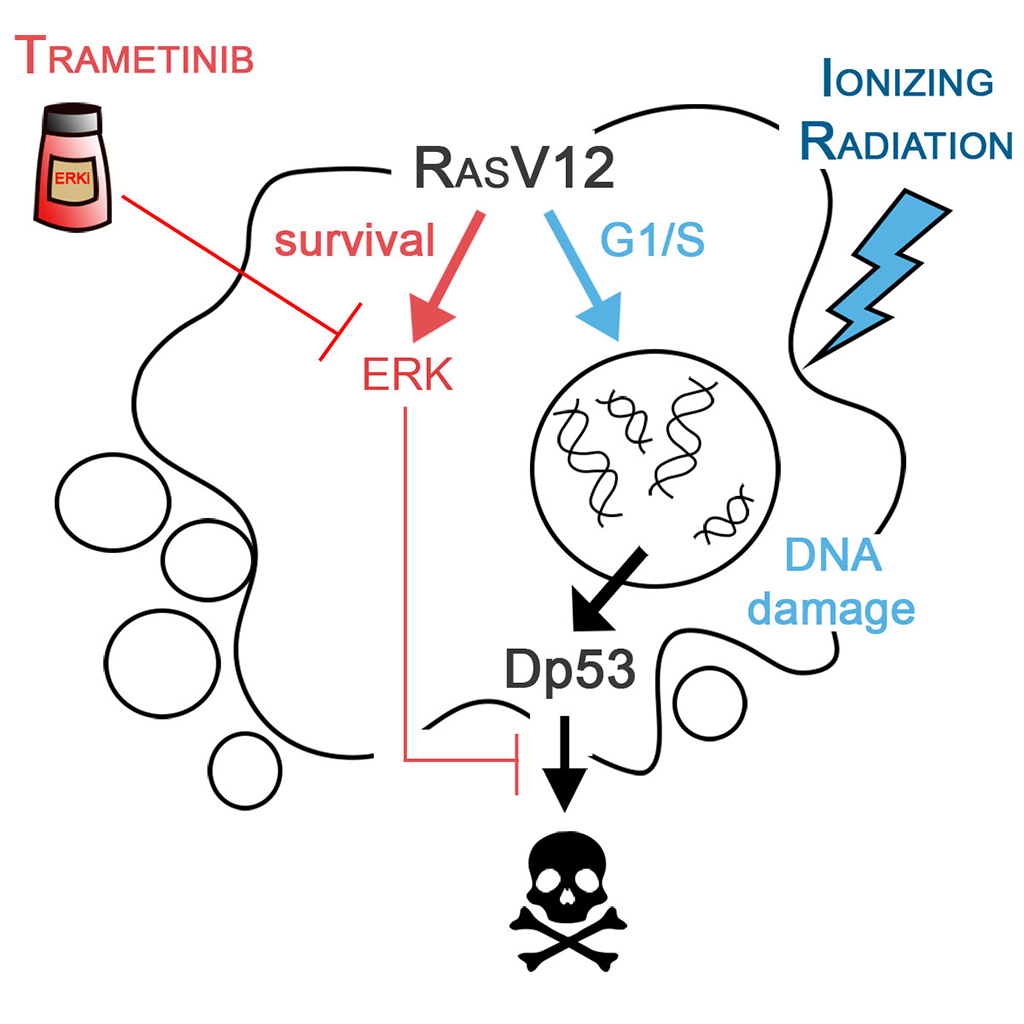
More than 30% of all human tumors arise from mutations that encode a RAS protein essentially locked in a constitutively activated form. Despite more than three decades of effort by Academia and Industry, only recently have effective RAS inhibitors started to be identified. We have used the fruit fly Drosophila as model system to identify strategies to specifically eliminate tumor cells that activate this oncogene. We have presented evidence that RAS drives active cell proliferation through the regulation of the G1-S transition, induces DNA damage, and most interestingly, silences the response to this damage by blocking the DNA damage response (DDR) pathway as well as the induction of cell death by the tumor suppressor protein Dp53. Given the inherent induction of DNA damage and additional blockade of the DDR at different levels by the RAS oncogene, we have used genetic or chemical inhibition of ERK activity coupled to ionizing radiation as a therapeutic approach to selectively eliminate RAS-malignant tissues. In particular, we have used TRAMETINIB, a drug prescribed for human melanoma, to inhibit the ability of RAS to block cell death, thus leading to the elimination of malignant tumors—selectively and by cell death—without affecting the development of organs or the flies themselves. Our results open up the possibility of combining radiation therapies with RAS inhibitors to selectively eliminate tumor cells.
Reference