In changing weather situations, plants need to be robust and flexible at the same time. These structural properties are anchored in the cell walls. The cell wall is responsible for defining the plant’s shape, for compensating its osmotic pressure and to protect it against pathogens e.g. bacteria, viruses or fungal attacks. The cell walls of plants are largely built from polymers and the polysaccharide cellulose. As binding agents, polysaccharides have the important task to connect longchain polymers and to build a molecular network of tiny strands, called fibrils, which contribute to the tensile strength of the plant.
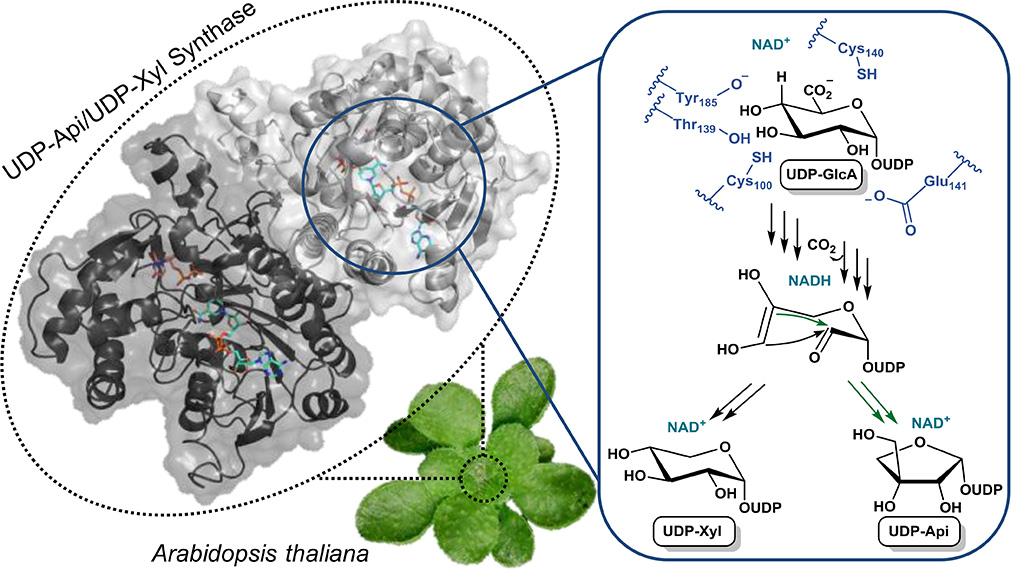
One of the sugar building blocks is the branched-chain monosaccharide apiose, which got its name from the latin word “apium“, a plant genus comprising, for instance, celeriac and parsley. Apiose has been investigated by plant-biochemical research for more than a hundred years and its function in plants is still not fully understood. Besides, the mechanism, which is responsible for the production of apiose in nature, was still unknown.
Scientists from the Universities of Barcelona (Spain), Pavia (Italy) and TU Graz (Austria) discovered how apiose is produced by a single enzyme called UAXS (UDP-apiose/UDP-xylose synthase). They were able to decode the entire mechanism of this enzyme for the first time. Isolated from the cress Arabidopsis thaliana, the catalyst possesses special properties: whereas most biosynthetic processes for the manufacturing of complex molecules need several reaction steps, the UAXS-enzyme selectively catalyzes in just four reaction steps. By doing so, the enzyme is able to break down organic carbon compounds as well as to establish new molecular compounds. This results in the change from a six ring sugar molecule (hexose) to a structurally simpler five ring sugar (pentose). By creating new organic carbon compounds, the enzyme is responsible for giving plants their strength properties.
The discovery of the enzymatic mechanism of apiose was possible due to the interdisciplinary collaboration between the research areas of molecular modeling, enzymology and biocatalysis and structural biology.
Reference