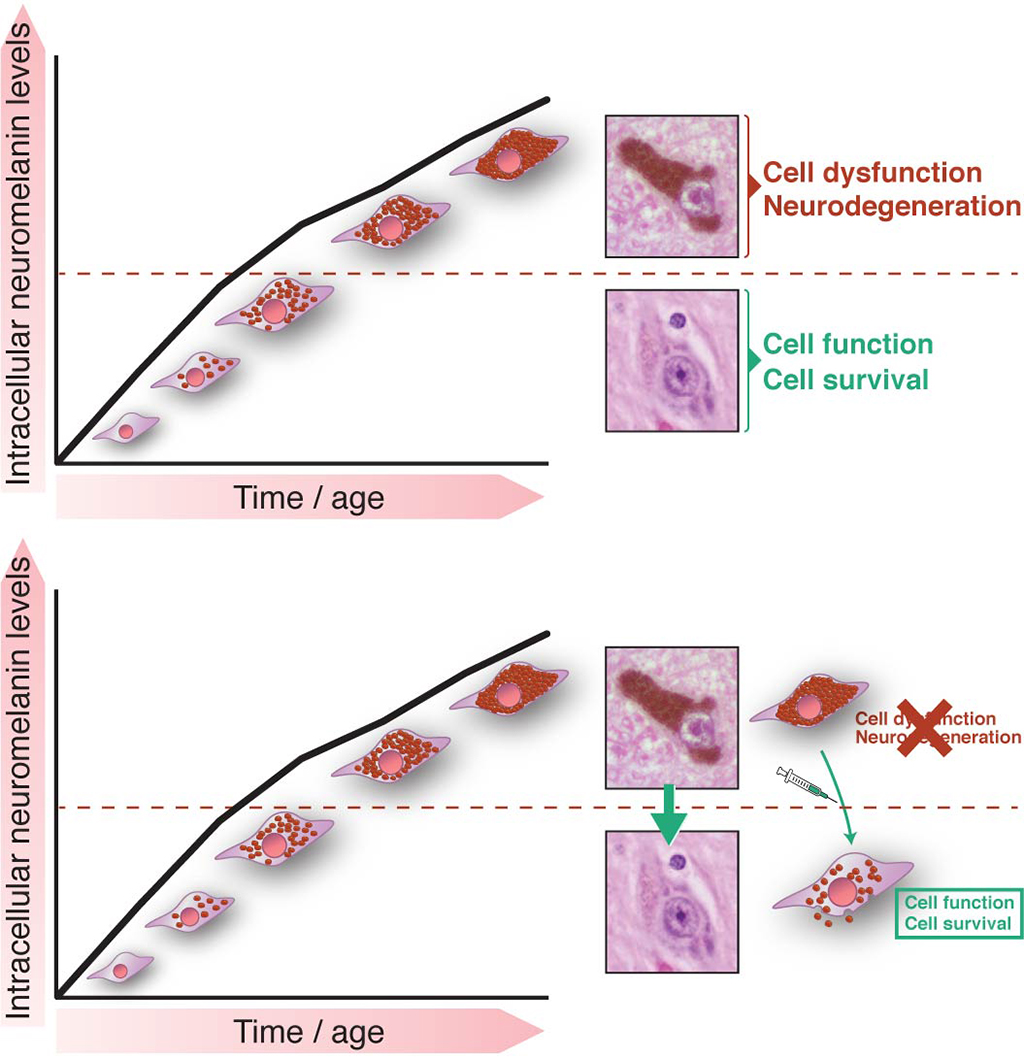
In Parkinson’s disease (PD) there is a preferential degeneration of neurons containing the dark-brown cytoplasmic pigment neuromelanin (NM), especially dopaminergic neurons of the substantia nigra the loss of which leads to the classical motor symptoms of PD. In humans, NM appears in early childhood and accumulates progressively with age, and increasing age is the main risk factor for developing PD. While the loss of pigmented nigral neurons in PD was first established a century ago and constitutes the cardinal pathologic diagnostic criteria for the disease, the potential contribution of NM to PD pathogenesis has remained largely unknown because, in contrast to humans, laboratory animal species commonly used in experimental research, such as rodents, lack NM. Thus, a factor so intimately linked to PD such as NM has been surprisingly neglected to date in experimental in vivo paradigms of the disease. To overcome this major limitation, we developed the first genetically-engineered rodent model exhibiting age-dependent production and accumulation of human-like NM within PD-vulnerable nigral neurons, at levels up to those reached in elderly humans. Using this unique animal model, we found that progressive intracellular build-up of NM with age ultimately compromised neuronal function when allowed to accumulate above a specific threshold, eventually triggering in an age-dependent manner all the main pathological features of PD. Relevant to humans, intracellular NM levels reached this pathogenic threshold in PD patients and pre-PD subjects but not in healthy elderly individuals. Importantly, the lowering of intracellular NM by gene therapy to levels below this pathogenic threshold reversed PD symptoms and pathology in NM-producing rodents. These results suggest that progressive, age-dependent intracellular NM accumulation above a pathogenic threshold might be responsible for the initiation of PD. Accordingly, strategies to maintain or decrease intracellular NM to levels below its pathogenic threshold may provide unprecedented therapeutic opportunities to prevent, halt or delay neuronal dysfunction and degeneration linked to PD and, in a broader sense, brain aging.
Reference