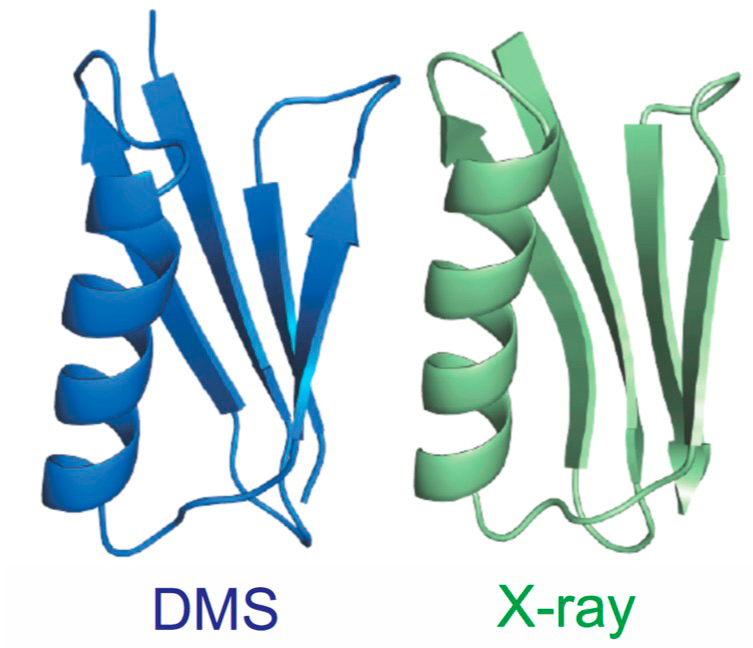
Determining the three-dimensional structures of macromolecules is a major goal of biological research because of the close relationship between structure and function. Structure determination usually relies on physical techniques including X-ray crystallography, NMR spectroscopy and cryoelectron microscopy. We have developed an alternative method that allows the high-resolution threedimensional backbone structure of a biological macromolecule to be determined only from measurements of the activity of mutant variants of the molecule. This genetic approach to structure determination relies on the quantification of genetic interactions (epistasis) between mutations and the discrimination of direct from indirect interactions. This provides an alternative experimental strategy for structure determination and one that allows the power of high throughput genomics to be applied to structural biology. It also allows the structures of molecules to be determined as they are performing their functions inside cells.
Reference