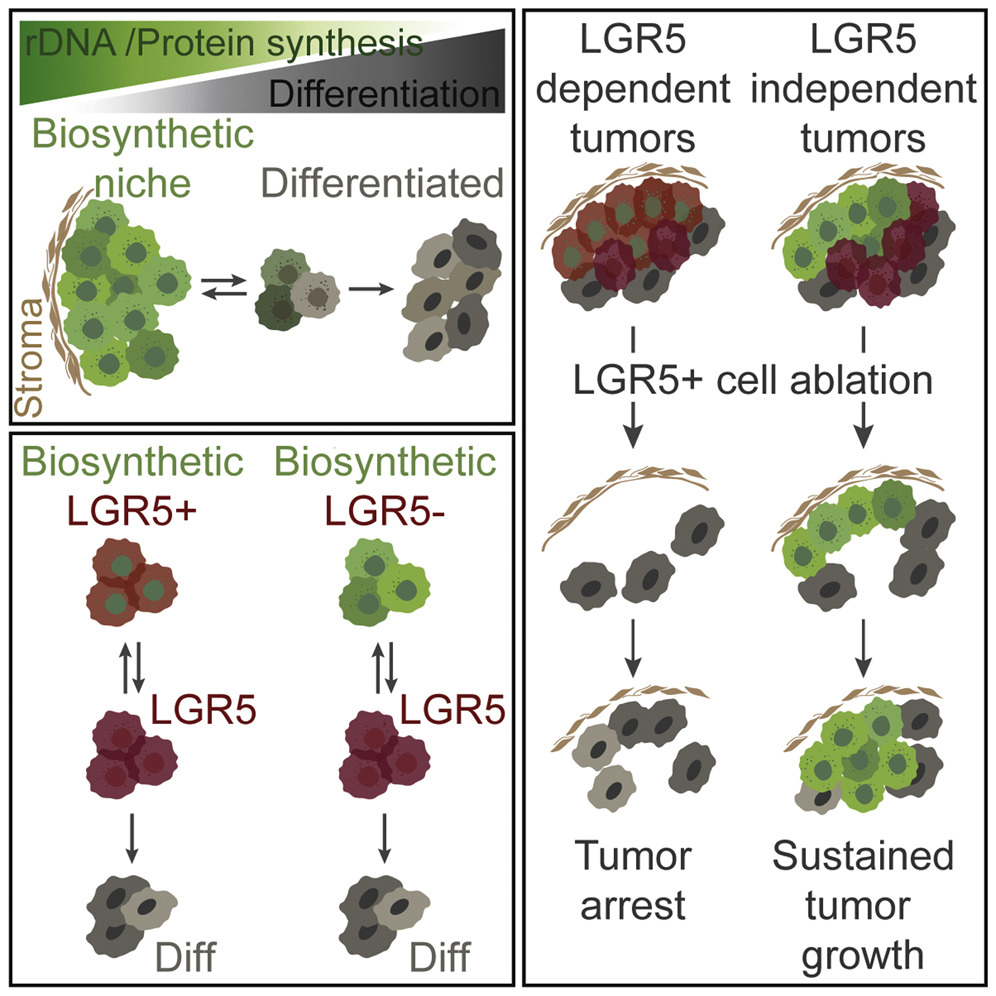
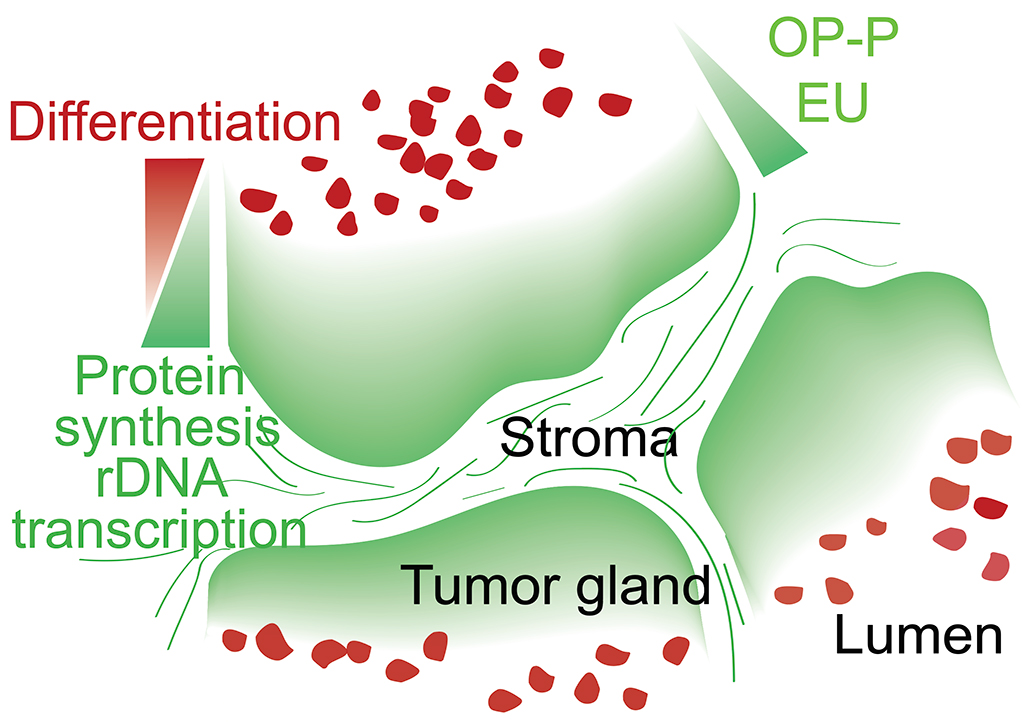
Tissue homeostasis requires controlled protein synthesis rates, and distinct cell types in healthy tissues exhibit different biosynthetic capabilities. In tumors, this regulation is disrupted by oncogenic alterations, many of which enhance the cell biosynthetic machinery, including ribosomal DNA transcription, ribosomal biogenesis, and protein production rates. We however discovered that the vast majority of ribosomal RNA and proteins synthesized in colorectal cancer (CRCs) are contributed by a limited subset of cells positioned immediately adjacent to the stroma. We showed that tumor cells that reside in these biosynthetic niches function as cancer stem cells although often they do not express LGR5 or other stem cell marker genes. In contrast, tumor cell differentiation is characterized by pervasive and permanent loss of biosynthetic capacities. To investigate the role of biosynthetic cells in CRC, we developed a methodology based on the use of CRISPR/Cas9 to edit the genomes of patient-derived organoids to introduce cell-specific ablation cassettes. Using this strategy in CRC models, we showed that lack of the biosynthetic cell compartment irreversibly halts tumor growth and induces tumor differentiation. We propose that zonation patterns of ribosomal DNA transcription and protein synthesis in CRC reflect the existence of a simple stem cell-like hierarchy based on the differential biosynthetic capacity of tumor cells. This model challenges some widely accepted views on the identity and features of stem cells in CRC and may inspire new therapeutic approaches.