One of the basic processes of nature is the decomposition of organic matter, which requires the degradation of complex carbohydrate structures. To do so, it is necessary to break the long chains of sugar molecules made up of smaller – ‘monosaccharide’ – units in order to transform them into short chains.
The above process is performed by glycosidases, enzymes found in all life forms and are part of the machinery by which cells acquire their nutrients.
In order to carry out these decomposition processes, only two catalytic mechanisms were known so far to help the acceleration of the corresponding chemical reactions. The most common mechanism is one in which the glycosidase enzyme uses two strategically located ‘amino acid residues’ to chemically cleave the bond. These molecules act like a pair of clippers that snip and cut the bond. This approach is used in a famous enzyme (lysozyme) found in egg-white that protects the chicken embryo from bacteria by cutting their cell walls.
The second mechanism needs uses one amino acid residue, and instead uses another type of chemical unit, an ‘amide group’ on the glycoside (such as in crab chitin) as the second residue. This is used by glycosidases called chitinases to degrade crab exoskeletons allowing them to molt. These are now textbook mechanisms studied in undergraduate courses.
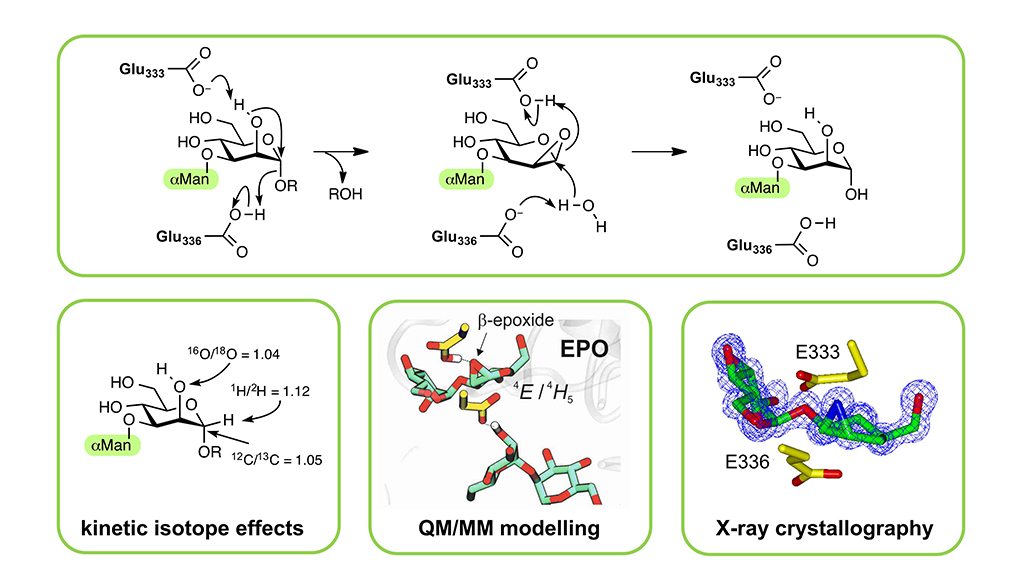
We have recently discovered a special group of enzymes that instead use another type of chemical structure, a sugar hydroxyl and that forms a three membered ring (an epoxide) intermediate. This mechanism was found in a specific type of glycosidases: endo-?-mannosidases, necessary to modify the sugars linked to our proteins.
The study has been carried out by an international team (UK, Canada, Australia and Spain), in which our group was in charge of modelling the enzyme mechanism of action using multiscale computational chemistry. The study provides the first convincing data to prove this particular mechanism from glycosidases, which will probably be used by other enzymes yet to be discovered. By learning how nature works, we can mimic its strategies to develop new enzymes for industrial applications.