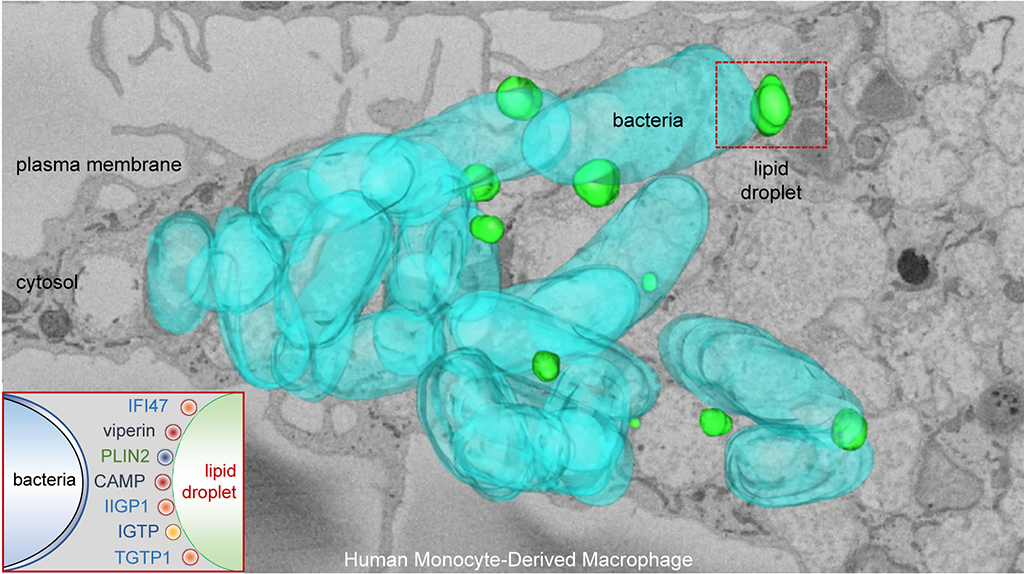
Background: In all eukaryotic cells, lipid droplets (LDs) store and supply essential lipids to produce signaling molecules, membrane building blocks, and metabolic energy. Common parasites (e.g. trypanosomes and Plasmodium falciparum), bacteria (e.g. mycobacteria and Chlamydia), and viruses (e.g. hepatitis C and dengue) induce and target LDs during their life cycles. The current view is that LDs support infection, providing microorganisms with substrates for effective growth. Rationale: Successful innate defense is critical for survival, and host species have efficiently co-evolved with pathogens to develop a plethora of immune responses. Multiple cues, including cellular stress and danger-associated molecular patterns such as lipopolysaccharide (LPS), induce LD formation. Thus, LD localization and dynamics may potentially be advantageous for organizing an intracellular host defense. Here, we have investigated the possibility that mammalian LDs have a direct and regulated role in innate immunity. Results: We show that mammalian LDs are endowed with a protein-mediated antimicrobial capacity, which is upregulated during polymicrobial sepsis and by LPS. In response to infection, multiple host defense proteins, including interferon-inducible GTPases and the antimicrobial cathelicidin, assemble into complex clusters on LDs. LPS additionally promotes the physical and functional uncoupling of LDs from mitochondria, reducing fatty acid metabolism while increasing LD–bacterial contacts. Conclusions: We demonstrate that LDs comprise a first line intracellular defense. They act as a molecular switch in innate immunity, responding to danger signals by both reprogramming cell metabolism and eliciting protein-mediated antimicrobial mechanisms. In view of the widespread resistance to current antibiotics, this study helps decipher molecular mechanisms involved in antimicrobial defense that could be exploited for development of new anti-infective agents.